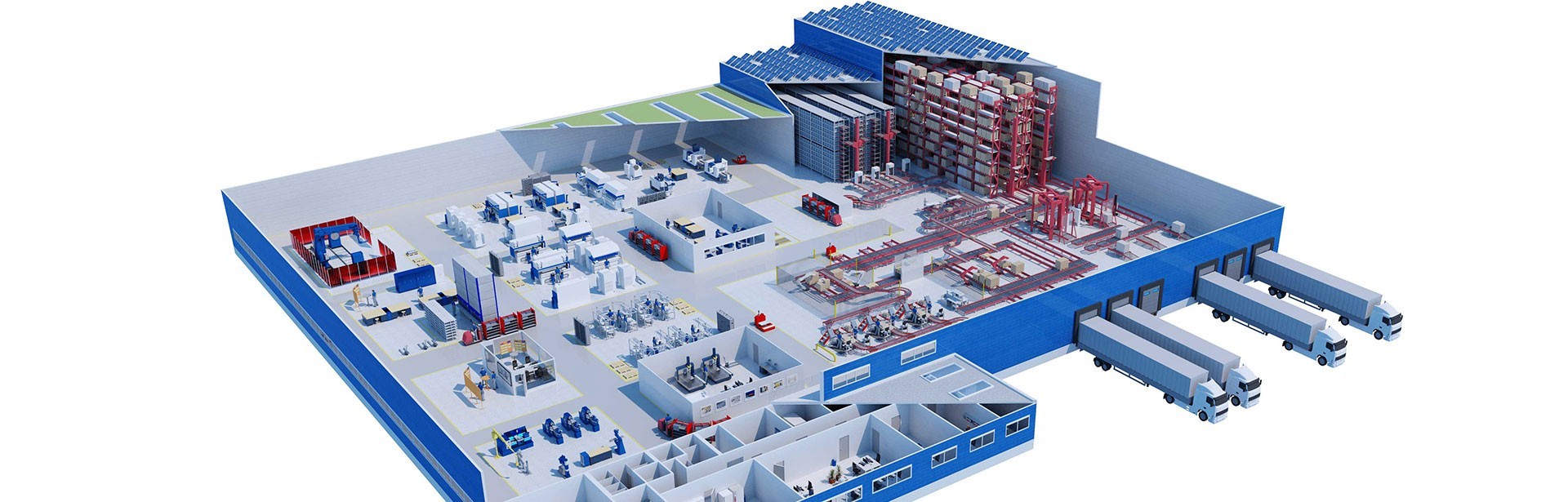
Merck’s Solida pilot plant goes live at Darmstadt
The GMP-compliant Solida pilot plant comprises a pharmaceutical solids manufacturing facility with OEB level 5 containment.
The production facility is unique in that it processes highly active substances that are dangerous to humans even at low concentrations.
All new elements had to be implemented in the existing building, taking into account the space limitations in production and the technical areas. io generated an integrated 3D model to incorporate all the involved disciplines into the planning, and subsequently saw through the implementation phase. The new production area at Merck’s Solida pilot plant has since been used for the research and development of innovative medicines.
The new pilot plant is without doubt one of the world’s most modern and safest solid-state production facilities for manufacturing highly potent medicines.
Key Facts
Client
Merck KGaA is a German chemical and pharmaceutical company with its headquarters in Darmstadt. Originally founded in 1668, Merck has gradually grown into a global company. Their workforce of around 57,000 people develops genome-editing technologies, discovers ways to treat diseases, and provides applications for smart devices.
The challenge
- Plan and implement a state-of-the-art solids pilot plant in a confined space in the existing environment, with potential expansion options in compliance with current GMP requirements and guidelines
Solution
- Develop and implement pharmaceutical solids production including containment up to OEB 5
- Use various granulation processes and closed transport systems (up to OEB 5)
- 3D planning of all sub-contractors including TGA